《鄉民大學問EP.38》直播|你反廢死嗎?歡迎來戰!廢死憲法法庭激辯!公投決定你支持?藍黨團提520後 邀賴清德立院國情報告!韓國瑜將正面對上 藍綠攻防?|NOWnews
NOW影音
更多NOW影音焦點
更多焦點-

黃珊珊批「北士科案」誤導視聽 蔣萬安酸:當事人不用太緊張
前台北市長柯文哲任內的北士科T17、T18地上權案延燒,台北市副市長李四川日前稱,T17、T18以「最低價」得標,引發前北市府團隊不滿,前台北市副市長黃珊珊痛批誤導視聽,並以內湖潭美段設定地上權案權利
2024-04-25 14:58
-

最高可貸200萬!勞動部助微型創業 她搖出中部首家24小時飲料店
為協助青年穩健創業,勞動部推動「微型創業鳳凰貸款」,可申貸額度最高200萬元,29歲的女青年金淑萍透過貸款,以及勞動力發展署中彰投分署的創業評估、研習、顧問諮詢輔導等一條龍服務,創立中部首家24小時手
2024-04-25 14:52
-

《淚之女王》金智媛結婚現場!韓劇CP御用飯店 玄彬孫藝真也愛這
金秀賢、金智媛搭擋的Netflix韓劇《淚之女王》,開播後一路維持高收視率,目前距離tvN電視台收視率第一的紀錄僅一步之遙。本週末《淚之女王》將播出完結篇,讓不少粉絲都十分期待金秀賢與金智媛這對CP可
2024-04-24 17:39
-

內湖三總驚傳死亡車禍!6旬婦人慘遭砂石車輾壓 緊急送醫仍不治
台北市內湖三軍總醫院門診大樓前,今(25)日上午驚傳一起死亡車禍,當時一名陳姓婦人(66歲)準備前往醫院抽血,不料經過院內道路時卻意外被砂石車撞上,直接失去生命跡象,雖第一時間送入急診室搶救,無奈仍因
2024-04-25 14:38
精選專題
要聞
更多要聞-

黃珊珊批「北士科案」誤導視聽 蔣萬安酸:當事人不用太緊張
前台北市長柯文哲任內的北士科T17、T18地上權案延燒,台北市副市長李四川日前稱,T17、T18以「最低價」得標,引發前北市府團隊不滿,前台北市副市長黃珊珊痛批誤導視聽,並以內湖潭美段設定地上權案權利
2024-04-25 14:58
-

世界閱讀日吳思瑤贈書 2藍委回贈、黃國昌不領情
本周二為世界閱讀日,民進黨立委吳思瑤表示,自己為化解司委會暴戾之氣,遞出橄欖枝分享好書,其中藍委羅智強最快回應,回贈自己的著作《靠岸》,另名藍委吳宗憲也回贈《文藝復興大師帶路》,完全抓住她的胃口,至於
2024-04-25 14:47
-

曾指陳時中認識詐騙角頭 徐巧芯大姑涉洗錢疑「牽扯柬埔寨詐團」
國民黨立委徐巧芯近日因大姑夫婦涉嫌詐騙,一言一行都遭到放大檢視,檢警持續追查,結果發現徐巧芯大姑夫婦躲在詐團金流第4層,還被查出機房設在境外,疑似和柬埔寨詐騙集團脫不了關係。對此,就有網友翻出徐巧芯過
2024-04-25 14:45
-

他點名藍「3大咖」不退場2028恐泡湯 藍委急緩頰:大家都有貢獻
前立委沈富雄日前直言,前總統馬英九、國民黨立委傅崐萁和國民黨主席朱立倫這「3大咖」若不退場,國民黨2028也不用想再戰。對此,國民黨立委萬美玲今(25)日《NOWnews今日新聞》節目《鄉民大學問》中
2024-04-25 14:08
新奇
更多新奇-

被《關鍵時刻》誤認成東大教授!有吉弘行看到了 本人回應超幽默
台灣政論節目《關鍵時刻》,由知名主持人劉寶傑領銜,時常針對各種時事進行語氣激昂的講解。不過近期在談論423地震話題時,誤將日本知名諧星主持人有吉弘行當成日本東京大學地震權威教授笠原順三,截圖瞬間在網路
2024-04-24 22:31
-
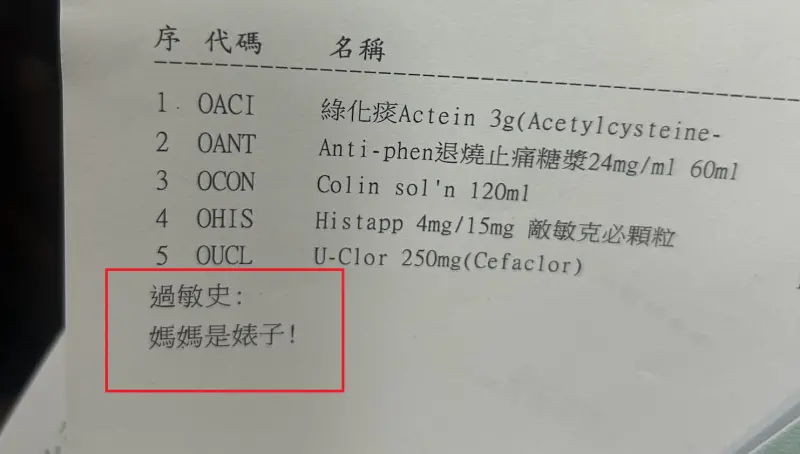
基隆醫院藥單上標註「媽媽是婊子」!人妻一看傻眼 護理師急道歉
(更新時間10:10)一位媽媽今(24)日帶著孩子前往衛生福利部基隆醫院看診,沒想到拿到藥單時,卻看到上面過敏史竟寫上:「媽媽是婊子!」讓她當場傻眼,詢問醫院究竟是怎麼回事,護理師同樣納悶,也立刻向她
2024-04-24 21:09
-

世界100大美食最新排行曝光!台灣竟成「吊車尾」:連英國都輸了
許多國際遊客來台一定會品嘗珍奶、小籠包、雞排、滷肉飯,甚至勇敢一點的還能嘗試看看臭豆腐。卻有國際美食榜單,將台灣美食排名列在榜單末端,甚至還有頗具權威性的美食評論刊物,2023年度最新榜單只給台灣第7
2024-04-24 18:43
-

全聯「稀有水果4顆99元」!隱藏福利曝:送保鮮盒 眾錯過再等1年
先別管60元便當了!全聯福利中心因為經常推出許多新品,或者是期間限定的產品,因此婆媽常常會在臉書社團上討論分享。然而近日就有不少人分享去全聯購買「寶石紅奇異果」,並且開箱分享口感,沒想到卻釣出內行婆媽
2024-04-24 18:29
娛樂
更多娛樂-

五月天甩假唱流言!北京鳥巢嗨唱10場 五迷憂:不想再看阿信吸氧
搖滾天團五月天為紀念出道25週年,舉辦「5525回到那一天」世界巡迴演唱會,昨(24)日所屬的相信音樂在粉專宣布,五月天將在5月18至6月1日重返北京鳥巢開唱,且一共唱10場,粉絲又開始擔心阿信的聲音
2024-04-25 13:20
-

丟丟妹沒運動1個月瘦20公斤!「減肥菜單公開」不復胖靠祕密武器
「直播天后」李明珊(丟丟妹)從爆紅之後,受到外界關注,有粉絲發現她從一開始臉蛋肉肉的形象,暴瘦到鎖骨顯而易見。近日她在IG上自曝,她靠著飲食控制,完全沒運動,一個月瘦下20公斤,令不少人稱羨,更透露還
2024-04-25 13:19
-

雷/《淚之女王》結局片花曝光!金智媛茫然探監金秀賢 劇迷崩潰
南韓影集《淚之女王》上週全國平均總收視率創新高,只差冠軍《愛的迫降》0.058%,可望榮登tvN電視台收視率最高的劇集,本週末將迎來大結局,官方搶先釋出1分12秒片花,金智媛到拘留所探監金秀賢,失去記
2024-04-25 12:28
-

《淚之女王》金智媛撞臉「J女郎」曾愷玹!4特質相似 對比照曝光
韓劇《淚之女王》自3月9日開播以來收視持續攀升,第12集播出後還擊敗《愛的迫降》成為韓國有線電視tvN電視劇在首都圈的收視冠軍,主演金秀賢、金智媛再度翻紅。J女郎曾愷玹也在追劇,她透露「最近也太多人跟
2024-04-25 12:18
運動
更多運動-

NBA季後賽4/25焦點球星/Tyler Herro英雄登場 SGA引領青春風暴
NBA在4月25日有兩場精彩的首輪季後賽賽事,本日的NBA季後賽主打球星,分別為邁阿密熱火隊的「英雄哥」Tyler Herro以及奧克拉荷馬雷霆的「SGA」Shai Gilgeous-Alexande
2024-04-25 14:43
-

不想要大谷翔平破自己紀錄!道奇主帥笑稱:我祈求球不要飛出牆
MLB美國職棒洛杉磯道奇今(25)日作客華盛頓國民,道奇打線全場狂掃20支安打,日籍球星大谷翔平也敲出大聯盟個人生涯首次單場3支二壘安打,終場率領道奇以11:2打爆國民。其中大谷翔平本場比賽個人的最後
2024-04-25 14:41
-

湖人再輸就沒退路!G3關鍵戰兩大悍將缺陣添變數 詹皇下達必勝令
NBA季後賽洛杉磯湖人隊將於明(26)日G3回到主場迎戰衛冕軍金塊,目前湖人已經在該系列賽陷入0:2落後,這一場比賽對湖人來說至關重要,但陣中前鋒Jarred Vanderbilt和中鋒Christi
2024-04-25 14:39
-

李亦伸專欄/熱火「區域聯防」教科書!切換自如、困死塞爾提克
熱火真金不怕塞爾蒂克,熱火G2客場111:101塞爾蒂克,七戰四勝系列賽扳成1:1平手。這是一場季後賽防守籃球教科書,熱火「區域聯防」,從1對1到輪轉,從強邊到弱邊,從包夾持球員到還原防守,不但讓持球
2024-04-25 14:23
財經生活
更多財經生活-

雨彈狂炸全台!開除濕機「省電3神招」必學 台電認證:每年省8%
近期台灣受到鋒面影響,北中南各地都有降雨機率,室內濕氣也因此暴增,除濕機開始長時間上工。那麼除濕機該如何使用才最省電呢?台電就曾點出3個必學祕訣,不僅可以每年省下8%用電,還能讓除濕機更有效運作,將室
2024-04-25 14:25
-
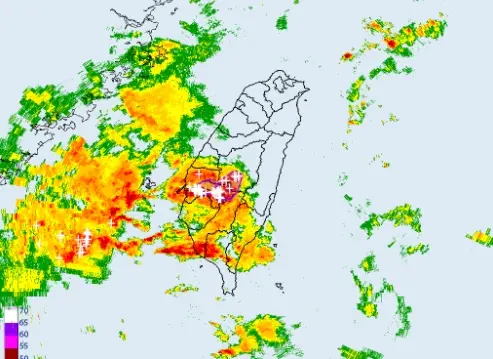
不斷更新/下班注意!高雄等3縣市「大雷雨警戒」 嘉義山區暴雨
今(25)日另一鋒面逐漸接近,天氣不穩定,降雨範圍廣,西半部、東北部地區及澎湖、金門、馬祖有短暫陣雨或雷雨,並有局部大雨發生。尤其是台中以南暴雨來襲,中央氣象署也發布「大雷雨即時訊息」和災防告警資訊,
2024-04-25 13:56
-

獨/高三生太強!開發地震APP、合作氣象署 800元地震站能裝你家
繼921後,0403花蓮7.2地震成為近25年來最強震,不斷餘震更搞的人心惶惶,紛紛尋求能提早得知地震消息的管道,2023年上路且廣受好評的「DPIP災害天氣與地震速報」目前擁有破10萬使用者,而在這
2024-04-25 13:23
-

彩券行老闆娘輸錯1號碼!幸運兒「爽中一等獎」 獎金秒翻2800倍
許多人喜歡買彩券碰碰運氣,看是否能中大獎,中國就有一間彩券行,因老闆娘手誤輸錯客人指定的彩券號碼,沒想到竟因此讓該名客人幸運中了一等獎,獲得獎金844萬元人民幣(約3795萬新台幣),比起原先沒輸錯的
2024-04-25 13:06
全球
更多全球-

日本自民黨再爆醜聞!已婚議員閃辭 被揭當乾爹頻點「外送茶」
日本執政黨自民黨籍眾議員、前防衛副大臣宮澤博行23日突辭去眾議員職務,傳出週刊將登出其醜聞,果不其然,日本知名爆料雜誌週刊文春隔日就揭露,已婚的且現年49歲的宮澤高調包養28歲年輕女子至租屋處,近期更
2024-04-25 13:56
-

日圓跌破155價位!「大拉麵指數」看遭低估74% 日銀干預機率增
日銀(BOJ、日本央行)在今年3月時睽違17年首度升息,但日圓匯率不升反貶,繼24日跌破155日圓價位後,25日開盤即貶破155大關,寫下34年來新低。美國金融科技公司DeepMacro引用「大拉麵指
2024-04-25 12:29
-

拜登又「凸槌」!競選演說疑似連讀稿機指令都唸 畫面瘋傳再惹議
高齡81歲的美國總統拜登,當地時間週三在華盛頓特區,出席北美建築工會(NABTU)的活動並發表演說,卻被抓包又出現言語上的失誤,疑似是看著讀稿機唸太順,結果連讀稿機指令也一併唸出。綜合《福斯新聞》、《
2024-04-25 12:24
-

華為新機AI修圖「一秒讓妹子變爆乳」惹議 公司承認:有漏洞
華為今年新機「HUAWEl Pura 70」本月18日正式開售,但近期被許多中國3C玩家和用戶發現,華為Pura70的「AI一鍵修圖」的功能,竟能讓原先穿著一般的妹子「一秒變低胸爆乳」,引發爭議。華為
2024-04-25 11:40