《鄉民大學問EP.38》字幕版|藍營2028的超級戰術!黃暐瀚:民調洩民進黨這弱點!廢死引爆民怨 是蔡英文挖坑給賴清德?台電屢跳電 萬美玲曝內幕:竟是小鳥惹的禍?!賴清德赴立院國情報告?將對上韓國瑜?
NOW影音
更多NOW影音焦點
更多焦點-

水源疑遭糞便感染!200萬瓶沛綠雅氣泡水銷毀 台灣家樂福先下架
礦泉水品牌沛綠雅(Perrier)水源疑遭「糞便」細菌污染,法國公共衛生機構命令銷毀200萬瓶氣泡水,台灣家樂福接獲通知,和頂級超市Mia C'bon皆已經預防性下架,而統一超商和全聯正進行內
2024-04-26 21:20
-

Google、微軟帶頭衝鋒!美股一掃Meta重挫陰霾 華爾街開紅盤
美國股市26日一掃停滯性通膨風險與臉書母公司Meta昨日重挫的陰霾,在Google母公司Alphabet與微軟財報強勁推動下,華爾街開出紅盤,截至寫稿時間為止,道瓊工業指數上漲132.54點、約0.3
2024-04-26 22:50
-

張書偉、謝京穎公布婚期!深V性感婚紗曝光 「一家4口」甜蜜合影
藝人張書偉、謝京穎2人去年12月已完成登記結婚,今(26)日公開婚紗照,並選定4個對他們十分有意義的場景,來拍攝2人的甜蜜婚紗。其中,張書偉、謝京穎2人還與寵物貓「哥哥」、「呀呀」共同拍婚紗,謝京穎的
2024-04-26 19:46
-
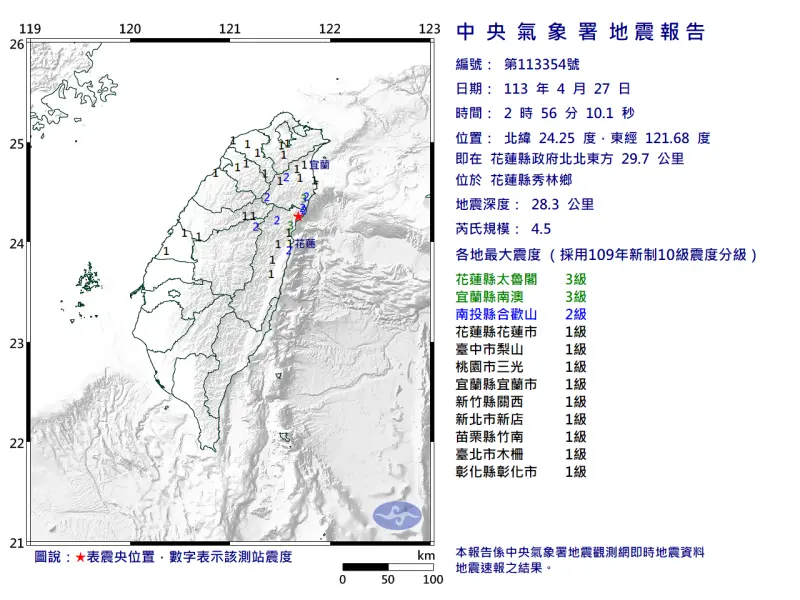
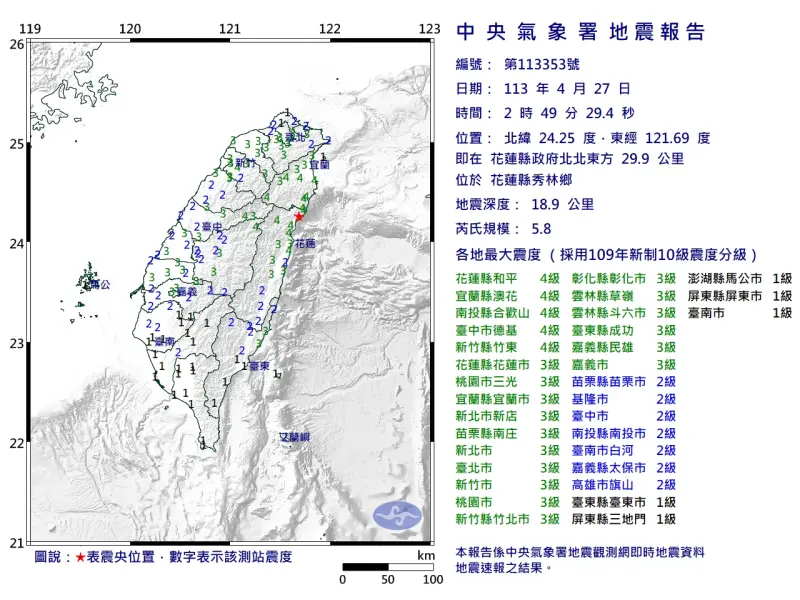
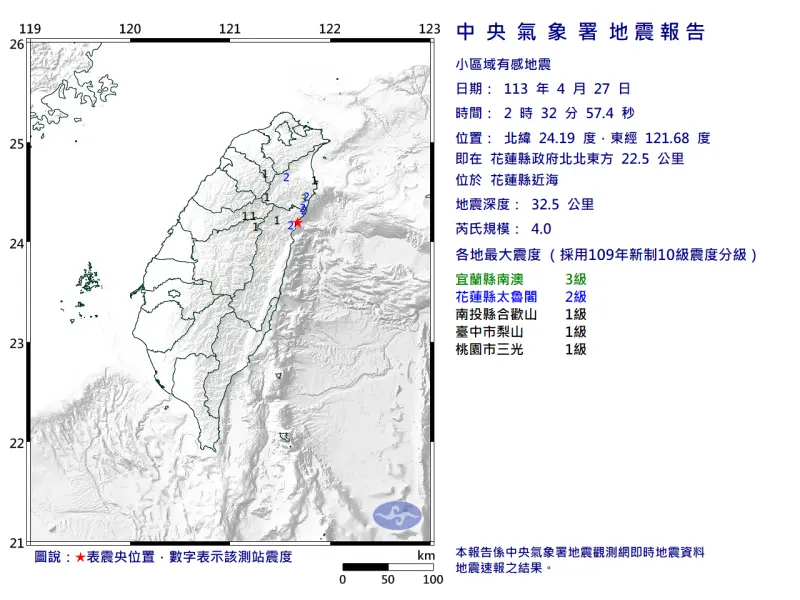
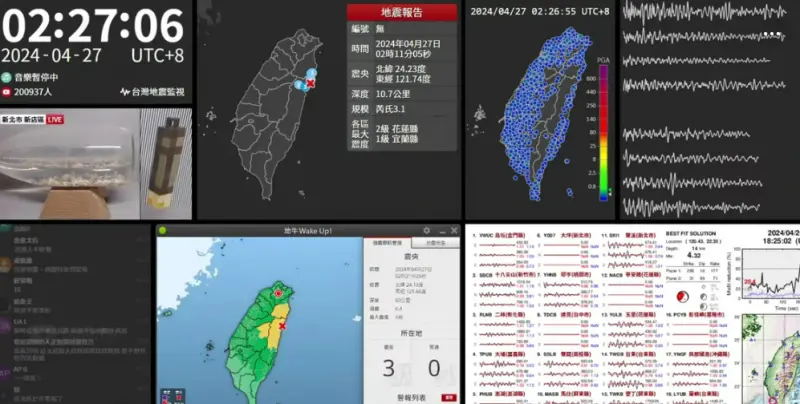
快訊/深夜震不停!02:56花蓮「規模4.5」極淺層地震 台北又搖了
地牛深夜不停翻身!據中央氣象署地震測報中心資料,今(27)日凌晨2時56分花蓮縣秀林鄉發生芮氏規模4.5地震,震央在花蓮縣政府北北東方29.7公里處,深度28.3公里,屬於極淺層地震。各地震度級花蓮縣
2024-04-27 03:07
精選專題
要聞
更多要聞-

中火以氣換煤遭疑偷渡5成發電量 藍黨團不滿將發動抗爭
台電推動中火以氣換煤計劃,地方原盼中部空污有望改善,未料台電新提出第二期新建燃氣機組計畫,赫見加上未來的4部機組後,可望將目前10部燃煤機組550萬瓩的發電量,增加到810萬瓩,儲氣槽也將由5個增加到
2024-04-26 18:46
-

寶林茶室監測結案!疾管署:通報35例造成2死、仍有4人住加護病房
寶林茶室食物中毒案自3月底爆發至今已有1個月,疾管署表示,至今通報35例、2人死亡、33例邦克列酸(原譯米酵菌酸)陽性、2例陰性,目前仍有4人在加護病房治療中。「寶林茶室信義A13店專案」通報專區,通
2024-04-26 18:11
-

范雲稱被黃國昌噴滿臉口水!民眾黨批「造瑤累范」:停止巨嬰行為
立法院今(26)日再度上演表決大戰立法院,民進黨團提案三案變更議程,其中與國會改革有關的兩案遭國民黨團提出異議,民進黨立委范雲在提案遭封殺後,不滿民眾黨團未對國會改革相關的反性騷擾案投票,與民眾黨團總
2024-04-26 17:47
-

影/卸任倒數!蔡英文玩賽車遊戲機當飆仔 狂飆160公里超開心
即將在5月20日卸任的總統蔡英文,今(26)日出席活動時大玩賽車遊戲機,「駕駛魂」噴發的她,笑容滿滿地直接狂飆160公里;被問到好玩嗎?她豎起大拇指大讚「非常好玩」;玩完賽車機後,她也玩起夾娃娃機,夾
2024-04-26 17:42
新奇
更多新奇-

台灣泡麵「隱藏王牌」爆出來了!每碗37元吃過上癮 老饕改買這碗
台灣泡麵在市面上販售的口味非常眾多,除了定期的新品之外,也有許多老字號的泡麵長年稱霸排行榜,成為台灣泡麵的一大特色。然而,近日就有網友分享一款台灣泡麵隱藏版,由於平常沒有在各大通路上架,因此只有內行人
2024-04-26 18:29
-

試管嬰兒站出來!婦產科蔡醫師迷因爆紅 本尊「是世界名醫」驚呆
2024最新迷因爆紅!最近在社群平台上,出現一名「活體迷因」,一位婦產科蔡醫師大爆紅,不僅有IG、Tik Tok的追蹤人數暴衝,甚至還有許多粉絲做了蔡醫師的各種周邊商品,由於過去蔡醫師還開投票讓粉絲表
2024-04-26 18:26
-

林叨囝仔七寶媽學歷曝!「最強外語」大學畢業 自嘲專長:生小孩
35歲的網紅「林叨囝仔」七寶媽(陳珮芬)歧視資源班言論遭炎上,不少人好奇七寶媽學歷為何?「七寶媽 學歷」在Google熱搜中快速飆升。據悉,七寶媽從小就是個學霸,國小到大學成績都名列前茅,她曾在部落格
2024-04-26 11:26
-

快訊/網紅七寶媽笑資源班遭轟!刪直播二度道歉:請原諒我的無知
網紅「林叨囝仔The Lins'Kids」七寶媽去年陷入多起爭議,好不容易才悄悄復出,結果日前直播上疑似嘲笑資源班的言論,讓她再度炎上。日前七寶媽Sydney深夜開直播道歉,但卻被批評是「把過
2024-04-26 09:15
娛樂
更多娛樂-

丁寧談七寶媽歧視!自曝小孩「就讀資源班」:感謝老師很能體諒
2024金穗影展今(26)日開幕,53歲女演員丁寧以短片《注意看,這個女人⋯⋯》主演身分出席活動,她在片中飾演的是包庇下屬肆意性騷擾的主管。丁寧提起近期演藝圈的各種醜聞,她表示:「其實這個在各行各業都
2024-04-26 22:54
-

「韓勾ㄟ金針菇」昔日賣泡菜被查出違規添加物!正式宣布重新出發
在YuoTube上擁有130萬人追蹤的人氣網紅「金針菇」去年在台灣推出韓式泡菜品牌「金家ㄟ」,吸引不少粉絲購買。然而去年「金家ㄟ」卻被查出泡菜內有違規添加甜味劑,讓她立即公告緊急回收所有泡菜,並向社會
2024-04-26 21:22
-

卜學亮談黃子佼!表態不會因此切割:做錯事要自己去承擔跟面對
黃子佼MeToo案持續延燒,同為「張小燕家族」的卜學亮今(26)日出席綜藝節目《我的明星村長》記者會時,被問及黃子佼事件,嚴肅地表示:「錯就是錯,我的看法就是當事人要自己去承擔跟面對。」卜學亮也被問到
2024-04-26 20:24
-

影/田馥甄到大陸開唱被轟:表態立場再來賺人民幣 女神高EQ回應
金曲歌后田馥甄(Hebe)今(26)日和林柏宏出席酒品代言活動,她5月將到大陸音樂節表演,部分大陸網友重提台獨疑雲,要田馥甄表態立場再來賺錢,對此,田馥甄低調表示:「能唱想唱的歌就覺得很珍惜。」▲田馥
2024-04-26 16:32
運動
更多運動-

NBA季後賽4/27戰報/KD率太陽主場拚開胡 快艇作客達拉斯搶G3
NBA在4月27日進行3場首輪季後賽的賽事,鳳凰城太陽回到主場迎戰明尼蘇達灰狼,這一場比賽太陽必須要拿下,「KD」Kevin Durant一直都能夠貢獻穩定的分數,但「奪命書生」Devin Booke
2024-04-27 00:05
-

澄清湖棒球場危機四伏仍開打?中職球員工會火大:請聯盟落實審查
4月24日在高雄市澄清湖球場舉行的二軍賽事中,由台鋼雄鷹出戰統一獅比賽,第一局就發現球場一壘側有異物,最後認定為鋼條,疑似為施工後未檢查到的遺留物,而高雄市運發局也主動發出聲明,並且致歉,但比賽已經從
2024-04-26 23:57
-

4/26NBA季後賽焦點球星/Aaron Gordon為湖人敲喪鐘!Embiid發威
4月26日NBA季後賽有三場比賽,主要由洛杉磯湖人出戰丹佛金塊,湖人在Aaron Gordon發威狀況下,最終以105:112不敵金塊,奧蘭多魔術以121:83擊敗克里夫蘭騎士,加上費城76人以125
2024-04-26 23:38
-

NBA季後賽/湖人面臨被聽牌絕境!詹皇喊話:活著就要堅持下去
詹皇信心喊話!NBA季後賽首輪G3由洛杉磯湖人在主場迎戰丹佛金塊,雖然湖人球星Anthony Davis攻下全場最高33分,仍於事無補,終場以105:115惜敗金塊。這是湖人自去年系列賽開始,對戰金塊
2024-04-26 21:52
財經生活
更多財經生活-

7寶媽訕笑資源班三度道歉!強調「這只是第1步」 下秒粉專消失了
網紅「林叨囝仔The Lins'Kids」7寶媽Sydney近日捲入失言風波,她在直播時被質疑恥笑、歧視「資源班」學生,隨後立即遭到炎上,並被多家廠商切割。7寶媽Sydney雖已二度道歉,但仍
2024-04-26 23:00
-

大樂透4/26頭獎槓龜!貳獎「狂中5注」平分 1人爽拿130萬獎金
大樂透第113000047期今(26)日晚間開獎,本期頭獎為1億元,但派彩結果顯示頭獎槓龜,貳獎則是有5注中獎,每注中獎獎金為130萬9299元,台彩保證下期頭獎仍有1億元。🟡4/26大樂透中獎號碼
2024-04-26 22:11
-

超哥新身分是Toyz同事!揮「超派鐵拳」3個月後認了:其實蠻內疚
網紅超哥(黃伯超)今年1月以「超派鐵拳」痛毆實況主Toyz(劉偉健),當時引起大量社會議論。超哥4月復出網紅事業,並成為Toyz直播平台的新同事,他近日以QA方式回應3個月前的暴力事件,坦言曾經感到愧
2024-04-26 21:01
-

快訊/大樂透頭獎保證1億!「4/26完整獎號」曝 中了秒財富自由
大樂透第113000047期今(26)日晚間開獎,台彩保證本期頭獎為1億元,就在剛剛完整獎號皆已開出,快拿起手邊彩券,看看能不能實現財富自由吧!🟡4/26大樂透中獎號碼(第113000047期)由小
2024-04-26 20:48
全球
更多全球-

Google、微軟帶頭衝鋒!美股一掃Meta重挫陰霾 華爾街開紅盤
美國股市26日一掃停滯性通膨風險與臉書母公司Meta昨日重挫的陰霾,在Google母公司Alphabet與微軟財報強勁推動下,華爾街開出紅盤,截至寫稿時間為止,道瓊工業指數上漲132.54點、約0.3
2024-04-26 22:50
-

越南國會主席辭職!助理甫因涉貪被捕 近1年已3位最高領導人下台
越南國會主席王庭惠今(26)日宣布辭職,是繼前國家主席阮春福、武文賞之後,1年多來第3位下台的越南國家級領導人,越南政壇打貪行動越演越烈,已經導致許多政府高層與商界人士落馬,包括貪污金額超過400億美
2024-04-26 21:42
-

H5N1禽流感在美國大流行!20%零售牛奶遭感染 專家擔憂病毒突變
美國食品藥物管理局(FDA)週四表示,最新發現有20%的美國零售牛奶樣本中含有禽流感病毒,樣本來自全美,不過FDA並未詳細說明樣本數目在各州的分布狀況,這似乎意謂著禽流感很可能已在美國大流行。根據《N
2024-04-26 20:41
-

台積電美國廠面臨「3大挑戰」!美媒:工作文化衝突未見好轉
台積電本月8日宣布獲美國晶片法案最高可達66億美元的直接補助,同時拍板美國亞利桑那州設立第三座晶圓廠,消息傳出後掀起熱議,不過對於台積電美國廠能否成功的擔憂從未減少,美國科技媒體《Apple Insi
2024-04-26 18:55