《鄉民大學問EP.36》字幕版|韓院長的突襲!藍綠白委員見“韓國魚”驚呼!謝龍介不敢睡 稱愈晚愈high在忙那椿?葉元之公開立院頭號女戰神是“她”!王世堅自豪這點連韓國瑜也比不上!|NOWnews
NOW影音
更多NOW影音焦點
更多焦點-

何佩珊掌勞動部 昔日老闆柯建銘喊:就看妳了!好好照顧勞工弱勢
準閣揆卓榮泰昨天宣布,勞動部長將由行政院副秘書長何佩珊接下,由於何佩珊過去曾擔任過民進黨立法院黨團總召柯建銘辦公室主任,柯也在臉書有感而發說,「佩珊接下來就看妳了,好好照顧勞工及弱勢」。柯建銘指出,何
2024-04-20 12:07
-

供電爭議頻傳!台電總經理王耀庭請辭道歉 經濟部慰留中
近來供電爭議不斷,傳出限電危機後又發生連日跳電,台電總經理王耀庭表示道歉,並不捨同仁遭外界批評,22日將正式向經濟部提出辭呈,經濟部長王美花則希望能慰留。台電和平電廠因0403地震造成損壞,原定1號機
2024-04-20 12:06
-

陳亞蘭久違扮花旦索吻!莊凱勛粉墨登場「擔心遭影迷追殺」
推廣歌仔戲不遺餘力的陳亞蘭,與莊凱勛兩位金鐘視帝聯手合作,共演歌仔戲職人劇《勇氣家族》,今(19)日舉辦開播記者會,在劇中飾演夫妻的陳亞蘭及莊凱勛盛重以歌仔戲扮相出場,過去反串男性角色居多的陳亞蘭,久
2024-04-19 18:18
-

快訊/今日氣溫飆破36°C!中央氣象署亮高溫燈號 示警地區一次看
今天(20日)環境轉為偏南到西南風,水氣減少,各地為多雲到晴、溫暖悶熱,午後在東北部地區及其他山區有局部短暫陣雨;氣溫持續回升,預估各地高溫可以來到31到34度,南部近山區及臺東有機會來到36度以上,
2024-04-20 11:47
精選專題
要聞
更多要聞-

何佩珊掌勞動部 昔日老闆柯建銘喊:就看妳了!好好照顧勞工弱勢
準閣揆卓榮泰昨天宣布,勞動部長將由行政院副秘書長何佩珊接下,由於何佩珊過去曾擔任過民進黨立法院黨團總召柯建銘辦公室主任,柯也在臉書有感而發說,「佩珊接下來就看妳了,好好照顧勞工及弱勢」。柯建銘指出,何
2024-04-20 12:07
-

酸沈伯洋專長是漆彈、帶風向 吳宗憲:看不懂專業法條才是笑死人
民進黨立委日前在立院司法法制委員會提案譴責召委、國民黨立委吳宗憲擔任主席但自己投下反對票,民進黨立委沈伯洋嗆「笑死」,前天更加碼嗆「誰沒念法律?」吳宗憲回擊,沈伯洋的專長就是漆彈、帶風向,「面對這種低
2024-04-20 12:04
-

北流1.9億爭議款案遭監院糾正 柯文哲喊話:蔣萬安加油
台北流行音樂中心1.9億爭議工程款案,監察院日前公告,認為台北市政府、文化部爭論不休,白白浪費利息支出「均有嚴重怠失」,均予糾正。對此,前台北市長、民眾黨主席柯文哲今(20)日表示,北流案是中央因為黨
2024-04-20 11:44
-

彭博社認證台灣要為停電做準備 王鴻薇:特定廠商壟斷讓國人受苦
總統蔡英文提出2025年2成電力來自可再生能源,但全球最大的財經資訊公司《彭博社》專欄作家高燦鳴(Tim Culpan)一篇分析指出,如果台灣政府的目標要利用保護措施來扶植本土風電產業,那麼台灣就必須
2024-04-20 11:39
新奇
更多新奇-

全美語幼稚園校外教學去資收場 家長氣炸:我小孩不會接觸這工作
孩子是國家未來的主人翁,不少幼稚園(幼兒園)會安排各項體驗活動,探訪警察局、走訪博物館,加深孩子對社會的了解。不過,一名家長近期抱怨,花大錢送孩子讀全美語幼稚園,結果校外教學居然去參觀資源回收場,質疑
2024-04-19 11:32
-

台大AV女優宣布徒步環台!首日狂走20公里「腳起水泡」 現況曝光
去年考上國立台灣大學的AV女優魏喬安,日前在社群平台上宣布要徒步環島旅行,第一天就狂走20公里,從台北車站走到三峽,也讓她腳上起水泡,再加上同行夥伴遭野狗咬傷,只能暫時回台北休養,不過她也強調,到下週
2024-04-18 20:05
-

一堆人到巴黎錢包被偷!黃大謙帶「8個錢包」實測 驚人結局曝光
法國的首都巴黎是擁有數千年歷史的古都,也是許多遊客到訪歐洲必去的浪漫城市。但當地治安卻總是亮起黃燈,一堆人都曾在當地錢包、財物失竊,當有人要去巴黎時,幾乎被提醒的第一句都是「小心錢包被偷」。對此,Yo
2024-04-18 18:38
-

佛心蛋「1顆賣1元」!每人限5顆 阿姨買100顆遭拒狂酸:還怕人買
不要糟蹋別人的心意!近期雞蛋價格雖然沒有去年「蛋荒」時昂貴,但想要買到一顆1元的雞蛋,仍可以說是天方夜譚。近期就有民眾表示,爸爸退休後迷上養雞、鴨,產生的蛋自家吃不完,就打算便宜販售,訂出「1顆蛋1元
2024-04-18 18:38
娛樂
更多娛樂-

人類淪為底層遭猩猩統治!《猩球崛起:王國誕生》解開物種之謎
《猩球崛起》系列電影,在全球獲得了16.8億美金(約新台幣504億元)的票房成績,而且每部都獲得奧斯卡視覺特效的提名;如今導演威斯柏被賦予新任務,就是在《猩球崛起:王國誕生》裡,讓猩猩在全新的大地上,
2024-04-20 11:42
-

謝和弦爆前妻Keanna噁心性事!正宮莉婭不忍全說了:跟過去和解
謝和弦前妻Keanna日前爆料,陳芳語(Kimberley)與前夫在錄音室吸毒並發生關係,陳芳語經紀公司強調:「內容純屬子虛烏有!」謝和弦則表示她記錯人,並爆料Keanna的噁心性事,令她震怒喊話回台
2024-04-20 11:12
-

劉亦菲「醉後模樣」全被拍!忘我撩裙又撥髮 零死角狀態極佳
藝人劉亦菲著名作品有電視劇《天龍八部》王語嫣、《神鵰俠侶》小龍女,因外型空靈有氣質被稱為「神仙姊姊」;近日,劉亦菲出席中國大陸上海時尚品牌活動,似乎是玩得有些開心,在After Party上喝到微醺的
2024-04-20 10:15
-

AV女優小倉由菜穿內衣大跳一粒「魔性舞」 葉保弟應援曲紅到日本
中華職棒台鋼雄鷹球員葉保弟的「魔性舞步」,因為台鋼雄鷹啦啦隊Wing Stars成員一粒(趙宜莉)而爆紅,吸引不少啦啦隊員及粉絲爭相模仿這首段舞。這股旋風也傳到日本去,讓知名AV女優小倉由菜都在自己的
2024-04-20 09:56
運動
更多運動-

NBA附加賽/Zion缺陣無妨!鵜鶘105:98退國王 搶下季後賽門票
美國職籃NBA今(20)日紐奧良鵜鶘在主場迎戰沙加緬度國王,雙方將要爭取西區最後一張通往季後賽的門票,勝者將以第8種子身分成功晉級,後續將要面對到西區龍頭雷霆隊,敗方則是本賽季確定結束。而本場比賽鵜鶘
2024-04-20 12:18
-

李智凱決賽難度分6.6、總分卻和預賽一樣 林育信發文:謝謝裁判
體操世界盃杜哈站暨奧運資格賽鞍馬項目,台灣「鞍馬王子」李智凱在決賽拿到總分15.400分的好成績,但卻不敵Ahmad Abu Al-Soud的總分15.500分,屈居銀牌,無緣在本站拿到巴黎奧運資格。
2024-04-20 12:00
-

T1/海神主控布銳克曼再見!先行返美養傷 幫隊友助威再拚總冠軍
T1高雄全家海神主控布銳克曼日前因左腳蹠骨骨折 (Jones fractures) 動刀,近日將先行返美,持續後續復健療程。布銳克曼感謝海神球團這段期間給予的醫療後援及協助,同時勉勵隊友面對季後賽保持
2024-04-20 11:53
-

MLB/Soto發威敲致勝3分砲!洋基5:3光芒 開季14勝6敗狀況火燙
美國職棒MLB紐約洋基在休息一天之後,今(20)日在主場迎戰坦帕灣光芒,前6局都沒有收下分數的「條紋大軍」,在第7局展開絕地大反攻,靠著重砲Juan Soto的一發三分彈在內,攻下5分大局,終場就以5
2024-04-20 11:09
財經生活
更多財經生活-

超商咖啡隱藏優惠!7-11美式、拿鐵「聰明買法」:比買1送1更便宜
7-11限時2天祭出咖啡隱藏折扣,那就是美式、厚乳拿鐵、精品美式、咖啡珍珠歐蕾、香鑽水果茶同品項第2杯10元,剛好可搭配滿額現折活動,指定支付方式滿888元現折100元,精品美式換算下來單杯比半價更優
2024-04-20 12:19
-
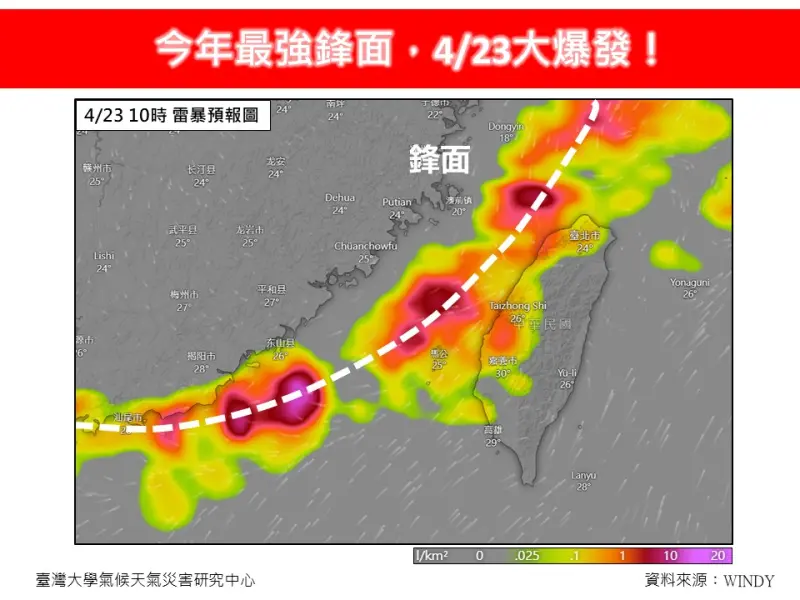
今年最強鋒面下週爆發!連6天「強降雨加雷擊」 專家示警恐釀災
今年最強鋒面要大爆發了!下週一(22日)起受鋒面接近影響,中部以北及山區轉雨,下週二(23日)日起雨勢最猛烈,對流發展旺盛時,常易伴隨短延時強降雨、雷擊及強陣風等劇烈天氣現象,至少會一路下6天,持續至
2024-04-20 11:35
-

不是台電的鍋!暨南大學跳電整晚 竟是「校內設備」故障
昨(19)日晚間暨南大學傳出跳電,有學生報告來不及存檔崩潰,台電表示,台電供電線路皆正常,是因為校內自有設備發生故障導致停電,已協助順利復電。昨日晚間10時,南投縣埔里鎮國立暨南國際大學傳出停電,台電
2024-04-20 10:36
-
蝦皮購物首支公益廣告上線 攜手公益團體推動百元捐款計畫
蝦皮購物昨(19)日上線首支公益廣告影片,宣傳與近百間公益團體合作推動的「百元捐」勸募計畫,傳達只要一百元、積少成多也能幫助弱勢族群的理念。蝦皮表示,自2018年起與公益團體合作線上勸募,一路上感謝公
2024-04-20 10:00
全球
更多全球-

史上第一人!台灣變裝皇后妮妃雅勇闖美《魯保羅變裝皇后秀》奪冠
四個月前,當多次獲獎的美國熱門實境節目秀《魯保羅變裝皇后秀》公布第16季名單時,一陣黃色炫風殺進了這個亞洲臉孔仍相對少數的圈子,來自台灣的變裝皇后「蕉佛」妮妃雅‧瘋(Nymphia Wind)頂著誇張
2024-04-20 11:00
-

伊拉克「親伊朗」軍事基地大爆炸!1死6傷 美國、以色列否認施襲
美國《有線電視新聞網》(CNN)、《路透社》20日引述伊拉克安全部門消息人士,聲稱位於巴格達南部巴比倫省的伊拉克民兵組織「人民動員」(PMF),其卡爾蘇軍事基地(Kalsu military base
2024-04-20 09:48
-

特斯拉開始被擠出中國市場!外媒專欄作家揭「養套殺」陷阱
電動車大廠特斯拉(Tesla)近期股價遭受打擊,除了裁員10%,還面臨來自中國新興電動車的激烈競爭。有外媒專欄作家分析,特斯拉的例子揭露中國對外商的「養套殺」模式,從一開始的市場誘惑,到中企的合資、收
2024-04-20 08:04
-
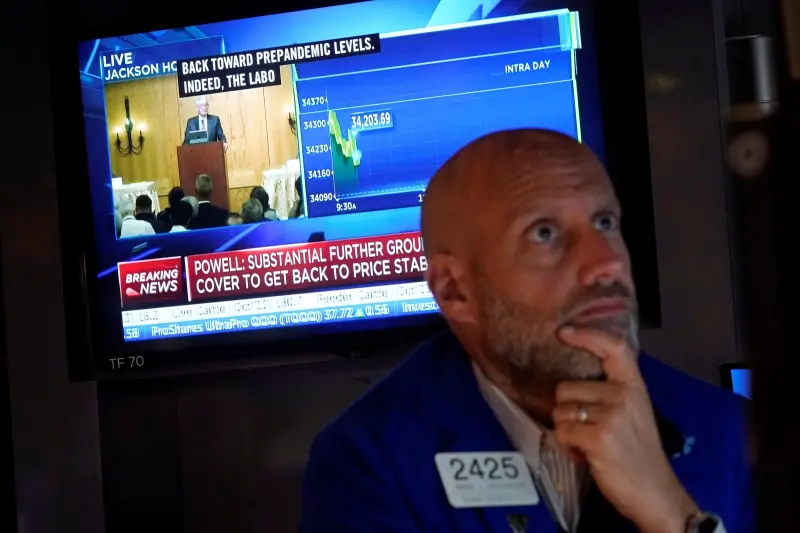
美股也迎黑色星期五?道瓊雖漲 納指大跌逾2%、輝達挫10%
伊朗淡化以色列的軍事報復行動,市場憧憬2國衝突應能避免進一步升級,美股主要指數週五個別發展,不過雖然道指收漲逾200點,但大型科技股受壓,納指下瀉超過2%,與標指都連跌6個交易日,顯示近期地緣政治衝突
2024-04-20 07:08