《鄉民大學問EP.39》字幕版|#柯文哲 的三大案連環爆!涉貪污遭列被告 陳智菡曝內幕!蔡正元:離總統路更近!民眾黨再演宮鬥劇?柯文哲與黃國昌竟是這關係?#韓國瑜 立院霸氣喊“閉嘴” 2028真再戰?
NOW影音
更多NOW影音焦點
更多焦點-

陳佩琪提早退休!嘆能力不符衛生局要求 蔣萬安:尊重職涯規劃
民眾黨黨主席柯文哲的妻子陳佩琪,在台北市立聯合醫院和平婦幼院區台北市長蔣萬安擔任醫師,昨日晚間她透過臉書發文,宣布要在今年9月提早退休,自認因為醫療專業和行政能力已不符合北市衛生局的要求,「做不下去只
2024-05-08 11:23
-

美股波動!台股高檔震盪有撐?高息主動式基金追求超額報酬
受到國際總經影響,近期台股波動劇烈,台股今(8)日開低,早盤一度跌百點,下殺到20547點,但午盤跌幅縮小,來到平盤附近。有專家指出,主要是市場對聯準會今年降息預期時程延後,高評價成長股進入重新訂價階
2024-05-08 11:10
-

周杰倫點名好友挑戰經典歌曲!林書豪演唱影片曝光:來個8.7分吧
「亞洲天王」周杰倫與前NBA球星林書豪的麻吉情眾所周知,2人時常在社群平台上互動,展現好交情。近日,林書豪在比賽時遭對手撞傷,導致右側眉骨撕裂傷。昨(6)日林書豪臉上頂著腫包,深情演唱周杰倫的經典歌曲
2024-05-07 15:47
-

嘉義惡火毀天倫!上週才提前慶祝幸福嗑鍋 媽生日當天遇火劫喪命
嘉義縣太保市民生路96巷一處平房昨(7)晚因不明原因發生火警,柯姓一家7口房屋全毀,其中母親、2名孩子因逃生不及命喪火窟,父親則抱著女兒衝出火場被濃煙嗆傷,目前仍在醫院插管治療中。從柯姓屋主的臉書可見
2024-05-08 11:16
精選專題
要聞
更多要聞-

蔡英文特赦扁幫賴清德拆彈?謝龍介爆最新內幕:「這事」還沒喬好
總統蔡英文520卸任倒數,不過有媒體報導指出,蔡英文已決定520卸任前特赦前總統陳水扁,並將針對扁家已扣案的11億餘元所得,全部聲請沒收。對此,國民黨立委謝龍介表示,這個議題是要幫準總統賴清德清理戰場
2024-05-08 11:17
-

徐巧芯炎上遭媒體逼問!傅崐萁再現身送暖:別攻擊國民黨認真立委
國民黨立委徐巧芯爆料外交部援助烏克蘭,被告洩密,他的財產仍持續遭檢視,質疑他房子買在京華城附近,投資精準。徐巧芯今(8)日現身立院受訪,而國民黨立院黨團總召傅崐萁也排在徐巧芯之後準被受訪,媒體連番問徐
2024-05-08 11:12
-

丟新事證強調沒洩密 徐巧芯反嗆吳釗燮:外交期刊都沒看多荒謬!
國民黨立法委員徐巧芯因曝光台捷密約,直指我國援烏專案透過第三方捷克,將援助金額轉入烏克蘭,但金流無法被監督,且恐有介入捷克內部政治疑慮,外交部更大動作提告徐巧芯洩密。對此,徐巧芯今(8)日再提出新事證
2024-05-08 10:56
-

徐巧芯喊「京華城關我屁事」 四叉貓爆:她2020年曾質詢該區都更
台北市京華城獲史上最高容積獎勵840%引各界質疑,而國民黨立委徐巧芯也被爆出3年前在京華城買了一間中古屋,被質疑靠內線消息獲利,不過徐巧芯對此直言「誰管他什麼京華城,京華城去死一死,我不管他今天容積多
2024-05-08 10:48
新奇
更多新奇-

變頻冷氣「5大天王優點」公開了!維修率最低這2牌:老闆認證推薦
炎炎夏日即將到來,不少人都開始準備替換家中老舊的冷氣,在知道冷氣怎麼吹省電之前,家中擁有一台好用效能好的冷氣更加重要!而說到變頻冷氣的一線品牌,一定會有許多人想到「日立」、「大金」、「三菱重工」、「國
2024-05-08 10:22
-

爸爸、哥哥、男友全都劈腿!「3人同1職業」女震撼:英國研究真準
身邊3個男人全部都偷吃!一名女網友最近發文分享抒發心情,表示自己的父親、哥哥以及現在的男友,職業全部都是「銷售業務」,然而全部共通點都是有劈腿的不良紀錄,讓她感到非常的無力直言:「我們家不知道是中什麼
2024-05-08 07:50
-

Energy坤達越老越帥!「21年前青澀照」出土 金秀賢也是同款男人
台灣男子團體Energy睽違20年合體復出,結果上週末在台北舉辦簽唱會,高人氣的他們竟然一路簽到凌晨三點,連團員坤達的老婆柯佳嬿都在社群平台上詢問:「簽完了嗎?」笑翻不少粉絲。然而,隨著Energy復
2024-05-08 07:11
-

全聯「隱藏任務」曝光了!結帳多1步驟倒賺40元 婆媽一看眼神死
全聯福利中心因為營業時間長,加上販售生鮮雜貨、日常生活用品應有盡有,成為不少上班族以及婆媽購物的首選之地,然而就有顧客最近去逛全聯發現,官方最近推出的最新活動,只要到全聯消費達到一定的次數、金額以及使
2024-05-08 06:23
娛樂
更多娛樂-

Energy當年不是坤達最紅?骨灰粉一面倒糾正「他才是」:妹子都愛
「最殺唱跳男團」Energy牛奶、阿弟、書偉、TORO、坤達2009年單飛不解散,相隔17年,本月初發行第七張專輯《HERE I AM》,不僅銷售開紅盤,兩場小巨蛋演唱會門票全被搶光,5日的簽唱會更重
2024-05-08 10:37
-

Energy「16蹲」舞步旋風全台!泌尿科醫師認證2大好處:性福加分
睽違21年,「最殺舞蹈男團」Energy終於再度合體,推出全新單曲〈星期五晚上〉展現「E16蹲」超狂舞步,引起眾多藝人和歌迷爭相模仿。然而,泌尿科醫師顧芳瑜也點出,勤練這款舞步的2大好處,除了可以讓「
2024-05-08 10:05
-

翁馨儀嫁張家11年「第一次和公公張菲合照」 親密勾手吐背後原因
31歲翁馨儀20歲帶球嫁大13歲張菲次子、同為演員的張少懷,結婚11年育有女兒「櫻桃」、兒子「栗子」。翁馨儀時常在粉專分享生活,不過鮮少提及公公張菲,昨(7)日她罕見公開和張菲的親密合照,居然是兩人首
2024-05-08 09:21
-

余天想養外孫「願支付開銷」 女婿Gary被爆情勒:先匯4000萬給我
余天女婿、次女余苑綺(已改名余泳澐)老公Gary(陳鑒)涉嫌和詐騙集團合作,擔任車手頭收受贓款,被依詐欺罪聲押獲准,余家上下聽聞Gary遭逮捕,第一反應就是去把兩個外孫接回來,據傳,余家在余苑綺癌逝後
2024-05-08 08:14
運動
更多運動-

KD有望加盟熱火?前NFL球星爆料:如果情況許可他會毫不猶豫來
本季鳳凰城太陽組建以Kevin Durant、Devin Booker、Bradley Beal為首的「三巨頭」陣容,雖打出49勝33敗、排名西區第6的戰績,但在首輪就遭到明尼蘇達灰狼直落四橫掃出局,
2024-05-08 10:34
-

灰狼能打破「最佳防守球員魔咒」?21世紀以來當屆DPOY僅2人登頂
明尼蘇達灰狼中鋒Rudy Gobert在今(8)日獲選本賽季NBA年度最佳防守球員(DPOY),為生涯第4次獲頒該將獎項,也加入了名人堂成員Ben Wallace和Dikembe Mutombo的行列
2024-05-08 10:19
-

NBA季後賽/Jaylen Brown轟32分!塞爾提克120:95大勝騎士拿下G1
波士頓塞爾提克今(8)日在NBA次輪季後賽G1迎戰克里夫蘭騎士,此役騎士一哥Donovan Mitchell狂轟全場最高33分,但「綠衫軍」靠著優異的團隊火力,Jaylen Brown、Derrick
2024-05-08 09:42
-

NBA季後賽/尼克中鋒Mitchell Robinson左腳踝再度受傷 將缺席G2
紐約尼克在季後賽次輪以121:117擊敗印第安納溜馬,但在第2戰開打前,尼克方面卻傳出壞消息,中鋒Mitchell Robinson因左腳踝傷勢,將不會出戰對陣溜馬的G2,Robinson的缺席對於尼
2024-05-08 09:31
財經生活
更多財經生活-

全聯177元「滅蚊神器」意外爆紅!婆媽見特價狂囤貨:蚊子不見了
炎熱夏天快來了!也代表著外面開始會出現許多小蚊蟲,防蚊、止癢成為民眾熱門討論議題,就有網友分享,睡覺時蚊子在耳朵旁邊嗡嗡叫超崩潰,剛好在全聯找到一款特價的液體電蚊香後,終於可以睡個好覺,熱心網友也認證
2024-05-08 10:52
-
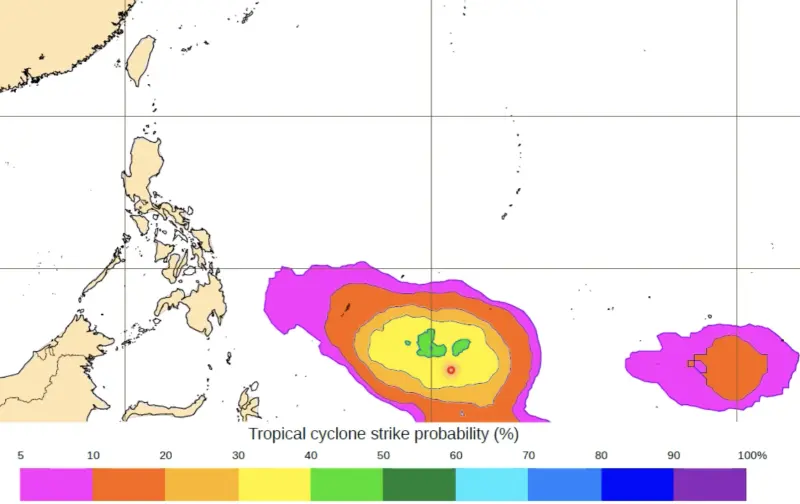
第1號颱風「艾維尼」要來了?賈新興揭生成機率 對台灣影響出爐
今年第一號颱風「艾維尼」(Ewiniar,米克羅尼西亞提供,原意為風暴之神)有機會在未來10天內生成,氣象專家、台灣整合防災工程技術顧問公司總監賈新興表示,目前各國預報模式分歧,以歐洲模式來看,在5月
2024-05-08 09:53
-

颱風「艾維尼」下週恐生成!今晚低溫跌1字頭 母親節後全台有雨
今(8)日東北季風增強、微弱鋒面通過,氣象專家、中央大學大氣系兼任副教授吳德榮表示,降雨主要集中在北海岸和東半部,山區午後有短暫陣雨,北台灣入夜氣溫驟降,低溫將來到攝氏19度左右,明後天(5/9、5/
2024-05-08 08:25
-

今起喝到母親節!7-11美式25元、拿鐵買1送1 全家寄杯奶茶買2送1
超商母親節優惠開跑!今(8)日起7-11美式、拿鐵、卡布奇諾買1送1,相當於濃萃美式單杯只要25元開喝,咖啡以外的品項則有黑糖珍珠撞奶、英式紅茶、青梅冰茶通通買1送1;全家則是限時3天推出私品茶買2送
2024-05-08 08:06
全球
更多全球-

巴哈馬承認巴勒斯坦為國家 稱「支持人民自由決定政治地位」
巴哈馬外交部7日發出聲明,宣布巴哈馬正式承認巴勒斯坦為一個國家,並稱支持巴勒斯坦人民自決,並重申支持兩國方案。巴哈馬外交部在聲明稿中表示,巴哈馬相信,承認巴勒斯坦國一事強烈表明,巴哈馬對《聯合國憲章》
2024-05-08 09:13
-

習近平的杯具頻受矚!訪法會談獨用「黑色水杯」 網:怕被下毒?
中國國家主席習近平近來展開五年來首次對歐洲國家的訪問行程,在結束對法國的國事訪問後,目前已經抵達塞爾維亞首都貝爾格勒。習近平這趟訪歐之旅,引起國際關注,除了到達法國時所承受的示威浪潮外,他在與法國總統
2024-05-08 08:44
-

台積電改變日本職場文化?鹿兒島企業嘆:年輕人跳槽門檻變低了
台積電於在日本熊本縣菊陽町設廠,成為近來台日工商業界的熱門話題。由於設廠帶來大量工作機會,有許多日本相關科系的學生,紛紛前往台積電日本廠求職。不過台積電熊本廠雖然帶來了錢潮,但也造成熊本縣其他企業面臨
2024-05-08 07:38
-

等重要經濟數據進入「冷靜期」!美股幾以平盤作收 道瓊連五紅
美國股市經過最近劇烈震盪後,在週二(7日)進入「冷靜期」,收盤接近開盤價。投資人正在等待將在本月公布的重要經濟數據。道瓊工業指數終場小幅收高32點,為連續第五個交易日收紅;標普500指數則為連續第四個
2024-05-08 06:28