《鄉民大學問EP.36》搶先看|韓國魚突襲立法委員辦公室!驚見不能說的秘密!謝龍介珍藏這個信物!王世堅突脫口:「你打過我們家的…」葉元之辦公室有美女助理群!范雲親教立院防身術|NOWnews
NOW影音
更多NOW影音焦點
更多焦點-

強震衝擊花蓮觀光旅宿業!經濟部5月推好市加倍券、夜市振興券
花蓮強震釀成嚴重傷亡,也波及觀光旅宿業,行政院會今(18)日討論「0403花蓮地震災後復原重建工作」,經濟部規劃將在5月起陸續推出好市加倍券以及夜市振興券以鼓勵民眾消費,並對花蓮4大商圈進行補助行銷,
2024-04-18 14:57
-

台股開低走高漲87點 外資持續提款66億、熱錢留不住?
美股四大指數昨(17)日全面下跌,其中又以費半重挫3.25%最重,拖累台北股市今(18)日開盤跌86.76點,台積電開盤跌破800元,不過10點過後,台積電股價拉升,帶動台股指數急拉,漲逾百點,盤中震
2024-04-18 15:08
-

謝和弦吐槽前妻Keanna記錯人!自爆親熱對象是「台灣天使」女優
謝和弦和前妻Keanna離婚4年,感情風波剪不斷理還亂,這次陳芳語(Kimberley)暗酸吳卓源欺侮人,沒想到Keanna跳出來大爆料,指控陳芳語與謝和弦在錄音室抽大麻偷情,遭到對方經紀公司嚴正否認
2024-04-18 13:26
-
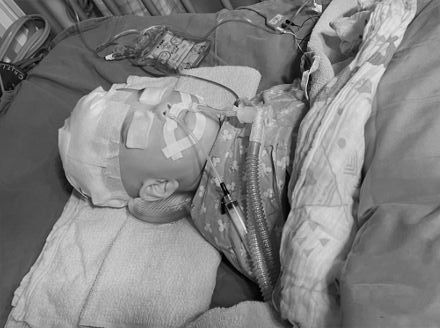
小桃子遭虐昏迷24天亡!惡保母只判9年 媽媽:對台灣司法好失望
台中市清水區1歲大女嬰「小桃子」(化名),2022年底送到蔡姓保母家托嬰,豈料卻出現顱內出血,送醫搶救24天不治,後續相驗後死因確認為「受虐性腦傷」,蔡姓保母涉有重嫌,依成年人故意傷害兒童致死等罪起訴
2024-04-18 14:25
精選專題
要聞
更多要聞-

偵辦寶林中毒案有重大進展?北市警局長曝:有重大發現應該會破案
遠百A13「寶林茶室」食物中毒案造成2死,震驚各界。北市府2日證實從寶林廚師的手部驗出邦克列酸毒素,全案檢警偵辦中,北市警局長張榮興今(18)日表示,此案有重大發現,可能會召開記者會對外說明,但檢察官
2024-04-18 14:50
-

桃園4度大停電 凌濤轟王美花自打臉:台灣已進入大缺電時代
桃園部分地區16日、17日出現停電狀況,台電解釋為配電線路事故問題,經濟部長王美花17日為此致歉,並強調「夏天前供電都會穩定」;沒想到今(18)日,桃園竟又再度發生停電情況,造成5,332名用戶停電。
2024-04-18 14:45
-

傳鄭文燦出任海基會董事長 綠委:賴清德對中遞出橄欖枝
總統當選人賴清德新內閣人事布局備受矚目,外傳行政院副院長鄭文燦將接任海基會董事長,對此民進黨立委吳思瑤今(18)日受訪時認為,鄭文燦風格理性且溫和,長期就是知中派,對中政策也是理性派,認為此布局也代表
2024-04-18 14:42
-

雙姝互嗆!陳玉珍批原民不重視離島 高金素梅反嗆:我也是有脾氣
立法院內政委員會今(18)日初審藍委高金素梅、鄭天財、陳玉珍提案《地方制度法》,不過最後陳玉珍的案子遭切割,另定期處理,引發陳玉珍不滿,陳玉珍還怒嗆召委高金素梅,不重視離島權益,高金反嗆,不接受這樣的
2024-04-18 14:31
新奇
更多新奇-

退休爸養雞賣佛心蛋!「1顆1元」每人限5顆 阿姨卻飆罵:怕人買
不要糟蹋別人的心意!近期雞蛋價格雖然沒有去年「蛋荒」時昂貴,但想要買到一顆1元的雞蛋,仍可以說是天方夜譚。近期就有民眾表示,爸爸退休後迷上養雞、鴨,產生的蛋自家吃不完,就打算便宜販售,訂出「1顆蛋1元
2024-04-18 13:48
-

政大景觀池命名票選!「金玟池」得票63%暫居第一 超紅原因曝光
韓流魅力真的太強了!近期國立政治大學要達賢圖書館旁的景觀池命名,更舉辦了人氣票選比賽,也開放校外民眾參加,沒想到除了許多諧音梗紛紛出籠之外,還有韓星「金玟池」的名字也在其中,甚至還突破6成得票率暫居第
2024-04-17 21:53
-

搭北捷親子友善車廂!她驚見「小孩全都站著」太諷刺 家長掀共鳴
台北捷運是雙北首都圈核心的大眾運輸工具,許多人上班、出遊都會搭乘到。近期卻有家長帶著孩子進入親子友善車廂,卻發現車上所有小朋友都沒有位置可坐,貼文隨即掀起大批家長共鳴。一位家長在社群平台Threads
2024-04-17 16:42
-

俄國妹領所有存款來台灣!向台籍男友求婚 霸氣喊「沒錢我養你」
為了追尋真愛勇氣十足!一位俄羅斯女孩Mila,透過交友軟體認識台灣男子,雙方迅速墜入愛河,後續Mila更直接帶了所有存款飛來台灣,還主動求婚,甚至霸氣喊「沒錢我養你」,主動追愛的過程曝光,也讓大批觀眾
2024-04-16 17:01
娛樂
更多娛樂-

日本樂壇Z世代鬼才imase宣布7/17登台開唱! 4/19快來搶票
日本樂壇Z世代鬼才 imase 看似平凡卻絕頂不凡,以洒落姿態與洗腦旋律在全球引爆現象級熱潮,imase 將於7月來台舉行首次個人演唱會,門票於明(19)日全面開賣。近期他也化身知名速食店大使推出新曲
2024-04-18 14:05
-

張小燕護航黃子佼有內幕!媒體人親揭「演藝圈生態」痛批還不解約
藝人黃子佼被爆出購買未成年少女影片後,遭到社會大眾撻伐,連師父張小燕也備受抨擊。媒體人兼作家莎莉夫人關注此事,先前曾發聲認為張小燕還護著黃子佼,希望她能公開呼籲黃子佼退出演藝圈,昨(17)日莎莉夫人再
2024-04-18 13:53
-

梁詠琪演唱會花海被酸像「告別式」 殯葬達人也傻眼:後勁太強了
香港天后梁詠琪出道28年,日前首度登上台北小巨蛋舉辦「時間遇上我們」演唱會,精選30首國語歌,她精心準備華麗服裝與舞台,不過其中一個花海場景卻讓人覺得怪怪的,連殯葬達人小冬瓜也說,「看到Gigi我感到
2024-04-18 13:49
-

傅娟攜3女上陸綜!歐陽妮妮被抓包「硬學大陸腔」 舌頭打結超尬
62歲藝人傅娟近日帶著3個女兒歐陽妮妮、歐陽娜娜、歐陽娣娣登上陸綜《是女兒是媽媽》,在節目中展現母女的互動、分享過往的生活點滴。近日歐陽妮妮談到妹妹歐陽娜娜曾自曝有容貌焦慮,其中一句「那你們又老讓她覺
2024-04-18 11:39
運動
更多運動-

MLB/大谷翔平躍升安打王!可望睽違14年 追逐鈴木一朗神紀錄
MLB洛杉磯道奇今(18)日在主場交手華盛頓國民,此戰道奇隊二刀流好手大谷翔平繳出三安猛打賞,是本次賽季第三次在同一場比賽中貢獻3支安打。開季至今已累積31支安打, 與隊友Mookie Betts、太
2024-04-18 15:00
-

橘爸唬籃/老鷹陣容已無成長性 送Trae Young去馬刺這主意如何?
NBA在昨天在西區附加賽打出了一場讓人感傷的結果,金州勇士遭到淘汰,讓外界感嘆王朝更迭。今(18)日東區附加賽結果也已經出爐,費城76人在老將Nicolas Batum的攻防俱佳表現帶領下拿到西區第7
2024-04-18 14:32
-

NBA/附加賽0分K湯想要頂薪!美媒:勇士恐留不住 獨行俠有興趣
隨著NBA金州勇士在昨(17)日附加賽被沙加緬度國王擊敗,除了宣告本季提前放暑假,陣中頭號射手Klay Thompson的去留也引起外界關注,其中《The Athletic》記者Sam Amick就在
2024-04-18 13:53
-

遭水原一平盜走5.1億沒差 大谷翔平砸5.4億在夏威夷買豪華別墅
MLB美國職棒洛杉磯道奇隊球星大谷翔平前翻譯水原一平涉賭案延燒近一個月,近日案情方明朗,美國聯邦調查局證實,大谷遭水源竊取1600萬美元(約5.1億新台幣),水原已被依詐欺罪起訴,若罪名成立恐將面臨八
2024-04-18 13:32
財經生活
更多財經生活-

恐怖炸物排行曝光!冠軍超意外「吸油率1500%」 專家:像顆油彈
鹹酥雞是台灣人宵夜首選,架上琳瑯滿目的品項,總是讓人看了食指大動。但營養師姚晴徽近日就統整出「油炸食物吸油率」列表,不只看似健康的蔬菜澱粉類,為隱形吸油熱量炸彈,炸海苔吸油率更是高達1500%,恐怖數
2024-04-18 14:47
-

日本工程師驚吐「台灣高鐵4祕密」!點名兩車廂最頂:商務艙同等
日籍正妹YouTuber「Mana」頻道擁有25.3萬訂閱數,她近日帶著爸媽來台灣體驗搭乘高鐵商務艙,她坦言自己住在台灣6年都沒搭乘商務艙,這次帶著工程師爸爸一起來體驗,爸爸也透露許多關於台灣高鐵的祕
2024-04-18 14:45
-

台積電好威!Q1 EPS 8.7元、優於市場預期 毛利率53.1%
台積電今(18)日公佈2024年第一季財務報告,合併營收約新台幣5926億4千萬元,稅後純益約2254億9千萬元,每股盈餘為新台幣8.70元(折合美國存託憑證每單位為1.38美元),優於市場預期。若以
2024-04-18 13:53
-

7-11店員超狂!新店型「現煮晶英牛肉麵、叉燒飯」 還能喝星巴克
超商店員無極限!近期統一集團旗下7-ELEVEN設立新店型,在台南永康打造7-11升級版的fresh店型,為全台首間有專人自煮餐飲的門市,不僅有台南晶英酒店現煮牛肉麵、叉燒飯、蔬食輕食,還有每日配送的
2024-04-18 13:26
全球
更多全球-

新一輪「太空競賽」?NASA署長:中國以民用太空計畫掩護軍事目的
美國航空暨太空總署(NASA)署長尼爾森(Bill Nelson)表示,中國透過民用計畫來掩護軍事目的,正在悄悄加強太空能力,華盛頓必須保持警覺。尼爾森是在出席眾議院針對NASA明年度的預算審議會議時
2024-04-18 14:56
-

馬斯克改抱印度大腿?外媒揭特斯拉將砸900億設廠
特斯拉(Tesla)執行長馬斯克(Elon Musk)將於22日訪問印度,與印度總理莫迪(Narendra Modi)會面,有外媒報導稱,馬斯克將在訪問印度期間,宣布投資20億至30億美元(約新台幣6
2024-04-18 14:19
-

杜拜機場遭世紀暴雨癱瘓!逾700架班機受影響 站內一團混亂
阿拉伯聯合大公國杜拜本周二遭遇暴雨襲擊,全球第二大運量的杜拜機場航班大亂,多達七百多架航班受影響,機場內也亂成一團。根據《路透》報導,周二這場破紀錄的暴雨,讓阿拉伯聯合大公國多數地區陷入癱瘓狀態,在杜
2024-04-18 13:04
-

美國會表決軍援案前 烏克蘭再示警:烏戰敗將爆發第3次世界大戰
美國眾議院將於本週六(20日)針對軍事援助法案進行表決,援助烏克蘭、以色列、台灣的法案將分拆成3案個別表決。其中軍援烏克蘭的610億美元能否過關,對於俄烏戰爭很可能帶來關鍵影響。對此,烏克蘭總理什米哈
2024-04-18 12:55